plasticker-News
| 2011-03-07, 06:15 |

|
DuPont: High performance polymers for use in medical devices
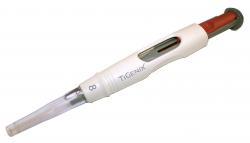 The ChondroMimetic™ from TiGenix NV is marketed as a procedure pack with the collagen implant preloaded in an accurate, easy-to-use arthroscopic delivery device. Specialty health care polymers from DuPont facilitate the smooth and accurate delivery of the implant for the user, whilst providing the material regulatory compliance for the manufacturer. Image: TiGenix The ChondroMimetic™ from TiGenix NV (Leuven, Belgium) is a CE-marked collagen based implant for the treatment of small osteochondral (cartilage and underlying bone) defects. The implant supports the body´s natural repair mechanisms to encourage the simultaneous repair of both articular cartilage and the bone to which it is attached. The product, which was officially launched in Europe during the 9th World congress of International Cartilage Repair Society in October 2010, is marketed as a procedure pack with the collagen implant preloaded in an accurate, easy-to-use arthroscopic delivery device. The device consists of several polymer components, including a white outer-casing made from ABS and a translucent polycarbonate delivery tube at its tip, which allows visual progress of the implant delivery to be maintained during the surgical procedure. Significantly, the two components made from Zytel® SC – the plunger and compressor fingers – come into direct contact with the implant and are key to holding it in place and inserting it smoothly and accurately into the defect site. The DuPont material was specifically chosen for the stiffness and low-friction behaviour of the plunger as well as providing the precise flexibility of the compressor fingers. “We specified a Special Control grade of Zytel® nylon based on the advice that the material comes with the required level of manufacturing control, testing and regulatory support for use in our delivery device,” explains Suhaib Hassan, product manager at TiGenix. “For instance, the device is required to meet the requirements of ISO 10993, which includes testing to support the storage of the implant in contact with the plastic parts of the delivery device for its entire shelf life.” Material supplier of choice to the health care segment DuPont offers a broad portfolio of engineering plastics and thermoplastic elastomers for medical devices, surgical devices and for diagnostic or pharmaceutical manufacturing equipment. The DuPont health care product offering provides food agency compliance (EMSA and FDA), ISO 10993-5 and -11 compliance as well as USP Class VI compliance. Speciality health care products are manufactured following GMP. The specialty health care grades with regulatory support are available in two versions, each reflecting different levels of manufacturing control and material testing. Customers work with DuPont to identify the version that meets the needs of their specific application. Sixteen of the products are available as “special control” grades that meet very high standards of manufacturing consistency important for a wide range of non-implantable medical products. Twelve of the grades are available in “premium control” versions meeting requirements for even more demanding manufacturing controls, broader regulatory support, more testing, DMF access and the highest level of inspection, says the company. More Information: www.dupont.com Medtec Europe 2011, 22.-24. March 2011, Stuttgart, Stand 4538 |
DuPont de Nemours International Sarl., Geneva, Switzerland
 back to news list back to news list |  back to top back to top |








